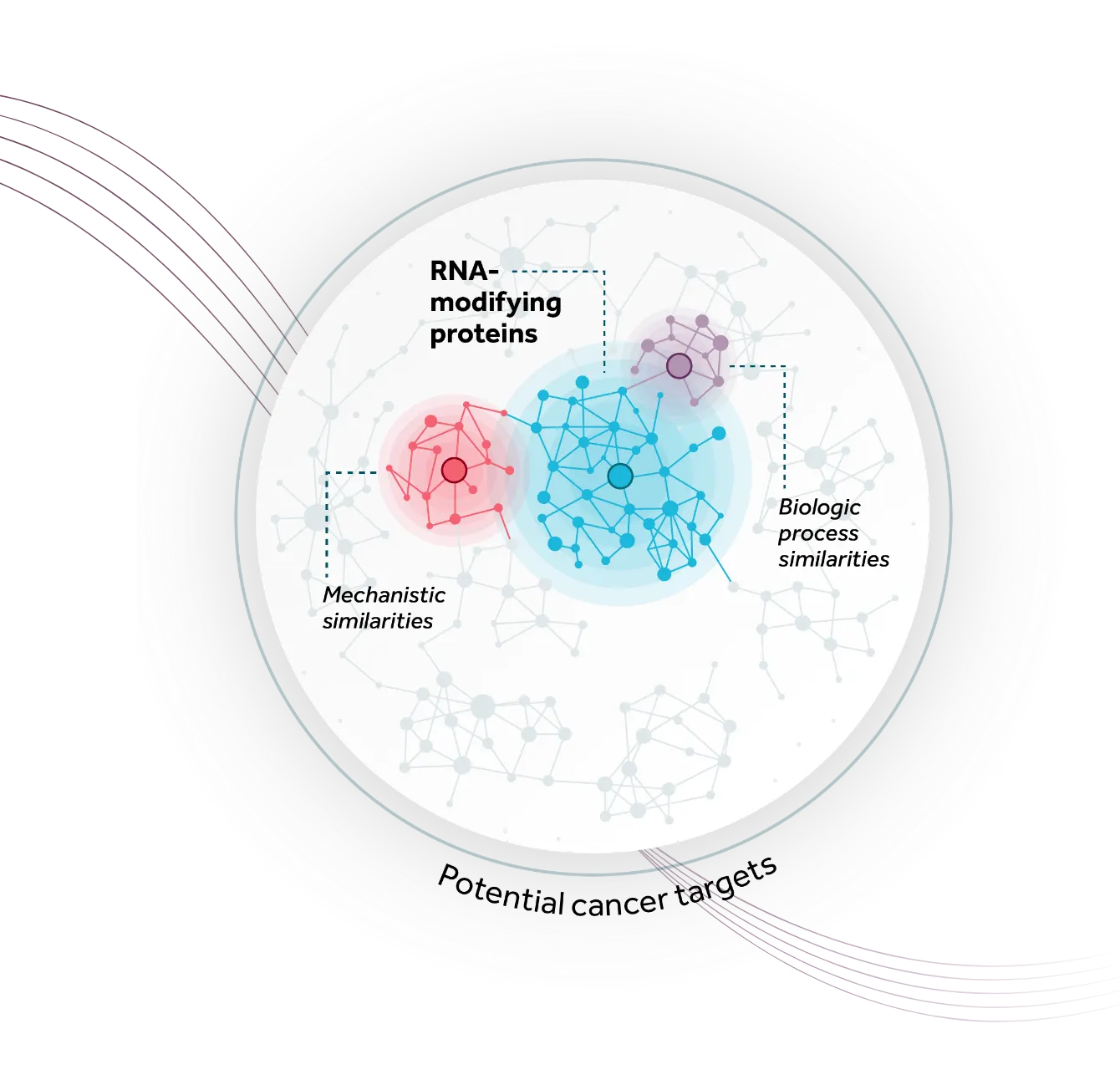
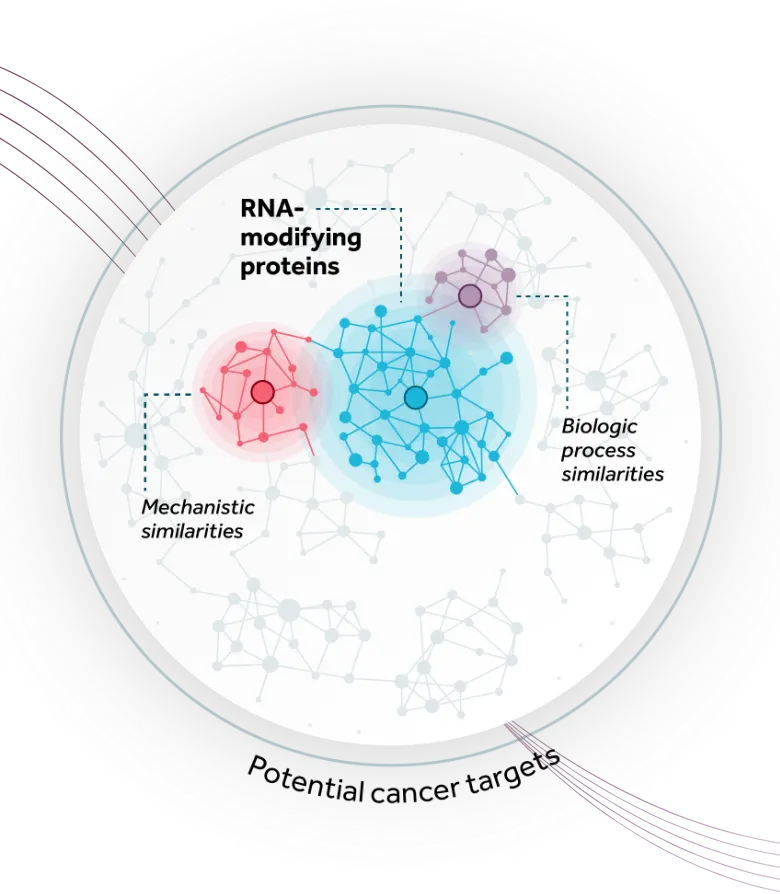
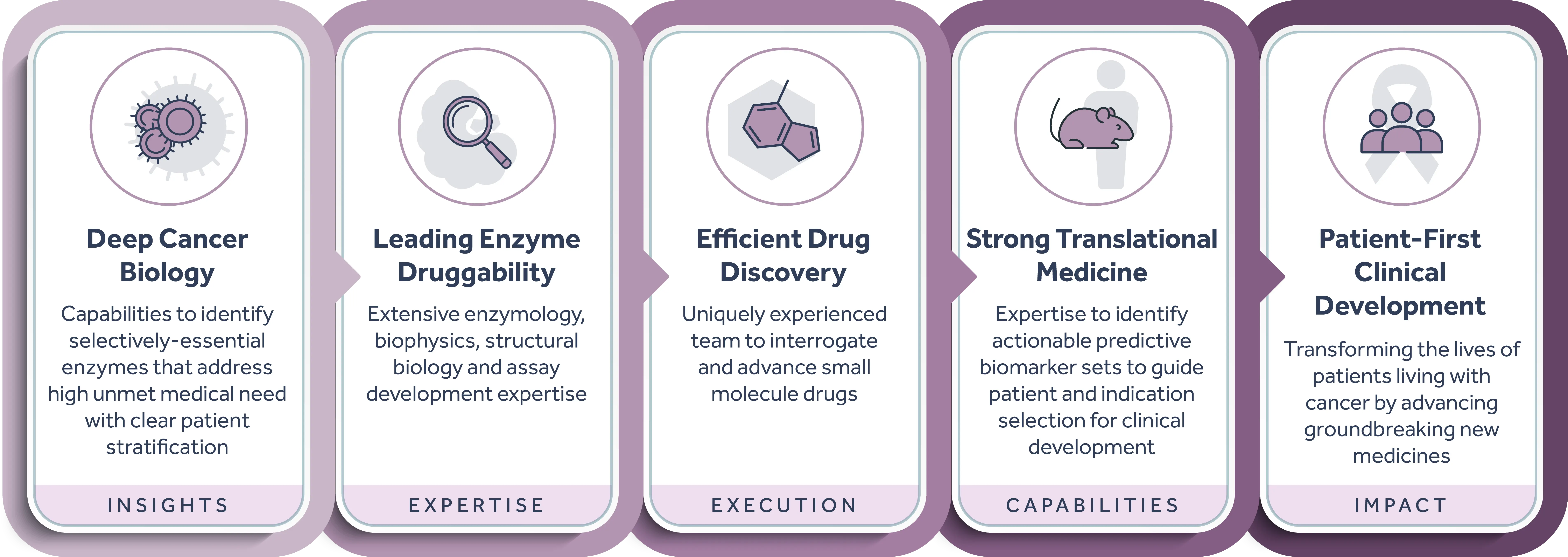
Accent’s lead program, ATX-559, is a first-in-class potent and selective inhibitor of DHX9, a novel and previously undrugged RNA and DNA/RNA helicase, shown to play a critical role in tumors with high levels of replication stress (including breast, ovarian, colorectal, endometrial, gastric, and others), representing large patient populations with significant unmet medical need. DHX9 has been reported to play important roles in replication, transcription, translation, RNA splicing, RNA processing, and maintenance of genomic stability, making it a compelling novel oncology target. In addition to exploiting key tumor vulnerabilities in DNA repair deficient backgrounds (e.g. BRCA) and hyper-mutated states (e.g. MSI-H/dMMR), Accent is exploring the sensitivity of other tumor types to DHX9 inhibition, and the potential to combine DHX9 inhibitors with other cancer treatments to maximize its full potential for helping patients.
ATX-559 is currently being evaluated in a first-in-human Phase 1/2, open-label, dose-escalation and expansion study, enrolling solid tumor patients with a focus on patients with BRCA1- or BRCA2-deficient breast cancer and those with MSI-H and/or dMMR solid tumors (including certain patients with colorectal, endometrial, gastric, and other cancers). To learn more, please visit www.clinicaltrials.gov (NCT06625515).
ATX-559 has been granted Fast Track designation by the FDA for the treatment of adult patients with unresectable/metastatic dMMR/MSI-H colorectal cancer post checkpoint inhibitor treatment. Fast Track designation is designed to facilitate the development and expedite the review of novel drug candidates that address serious conditions marked by unmet medical need, with the aim of accelerating patient access to novel treatment options.